The Lancet · Saturday 20 April 1968
Mortality and Hardness of Local Water-Supplies
MARGARET D. CRAWFORD, M.D. Glasg. and
M. J. GARDNER, B.Sc. Durh., Dip. Math. Stat. OF THE SCIENTIFIC
STAFF
J. N. MORRIS, D.Sc. Lond., F.R.C.P., D.P.H., HONORARY
DIRECTOR
MEDICAL RESEARCH COUNCIL’S SOCIAL MEDICINE RESEARCH
UNIT, LONDON SCHOOL OF HYGIENE AND TROPICAL MEDICINE, LONDON
w.c.1
Summary: In the sixty-one
county boroughs of England and Wales with population 80,000 or
over in 1961, the harder the local drinking-water and the more
calcium it contained the lower was the death-rate in middle and
early old age; this was particularly so for cardiovascular and,
to a lesser extent, bronchitis mortality, confirming findings
relating to the period around 1951. No evidence was obtained that
water hardness was reflecting some other environmental factor. A
multiple regression study showed that water calcium makes a
substantial and highly significant contribution to the variance
of the cardiovascular death-rate, between the sixty-one towns,
after allowing for environmental factors. Chemical studies of
trace elements in water from consumers’ taps showed none at
a concentration which could be considered toxic either in towns
with very soft or with very hard water. Apart from the main
minerals—calcium, magnesium, and sodium—only six
elements showed consistent differences between the soft and hard
waters—manganese and aluminium (higher concentrations in
soft waters), and boron, iodine, fluorine, and silica (higher
concentrations a hard waters). There is no acceptable explanation
at present for the associations found between water hardness and
mortality. There is, however, urgent need for incidence and
prevalence studies, and detailed investigation of the problem by
chemists.
Introduction
Epidemiological studies in several countries have shown that
death-rates from cardiovascular disease are higher in areas with
soft than in areas with hard drinking water (Kobayashi 1957,
Schroeder 1960a and b, 1966, Morris, Crawford, and Heady 1961,
1962, Biorck et al. 1965). In the eighty-three county boroughs of
England and Wales there were highly significant negative
correlations between cardiovascular mortality (1948-54, the "1951
series“ in middle age and contemporary estimates of both
total hardness and calcium content of local drinking-water, the
lower the death-rate. No such associations were found for other
causes of death, apart from bronchitis for which the correlations
were lower). In spite of an extensive search, no social or other
environmental factor associated with water hardness was found
that might “explain” these findings (Morris,
Crawford, and Heady 1961, 1962).
Material
A further study (the “1961 series“) has now been
made in the sixty-one county boroughs of England and Wales with a
total population of 80,000 or over at the 1961 Census; the
smaller county boroughs were excluded to reduce error in
estimates of the death-rates due to small numbers. The data
consist of 7 years’ mortality, 1958-64, using
cause-of-death groupings from the Registrar General’s
Abridged List, for men and women aged 45-64 and 65-74. A wide
range of social indices for each town was also assembled, mainly
from the 1961 Census. Chemical analyses of water as drunk locally
(i.e., after any chemical treatment of the supply) were obtained
for each town, including estimations of calcium (as
Ca++ magnesium (as Mg++ and sodium (as
Na+). Average values of hardness and of the main
minerals were calculated for each town; where there was more than
one supply in a town these were weighted by the proportion of the
population served to give a weighted figure.
Results
Table I shows the correlations between water hardness and
mortality for all sizeable causes of death in middle and early
old age (the product moment correlation coefficient is used
throughout as a measure of association). There are again highly
significant negative correlations with deaths from all causes in
the 4 sex and age groups studied—i.e., the softer the water
the higher the death-rate. Correlations with death-rates from
cardiovascular disease, which constitute over a third of total
mortality in both the male and female groups, are the highest;
mortality in the components of the group—cerebrovascular,
coronary, and other heart-disease—is also highly associated
with water hardness. Correlations with “all causes less
cardiovascular disease” are substantially lower, but in
contrast to the 1951 series (Morris, Crawford, and Heady 1961)
the coefficients are significantly different from zero.
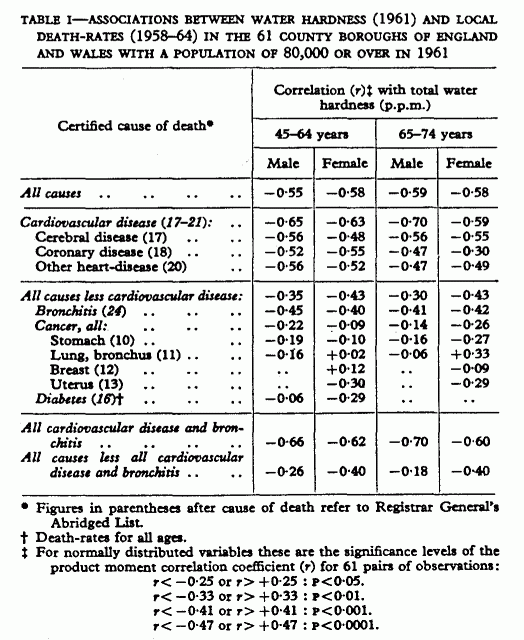
In both series, bronchitis is the only sizeable
non-cardiovascular cause of death at ages 45-74 showing
consistently high correlations with water hardness. In view of
the frequent cardiac complications of bronchitis and the
considerable interchange of this diagnosis with “myocardial
degeneration” on death certificates, this correlation had
not been considered previously to contradict the specificity of
the association between water hardness and cardiovascular
disease. The correlation coefficients of water hardness with
mortality from cardiovascular disease and bronchitis together
(table I) are much higher than those with all “other”
mortality, though these are also negative; these differences are
significant in the male but not in the female groups where the
correlations with “other” mortality remain high. Of
all the individual causes in this “other” group with
sufficient numbers to study separately, cancer of the stomach and
uterus have consistent negative correlations with water, though
of a different order to those of cardiovascular disease.
In the ensuing tables only the results for the 45-64
age-groups are shown; findings in the older groups are very
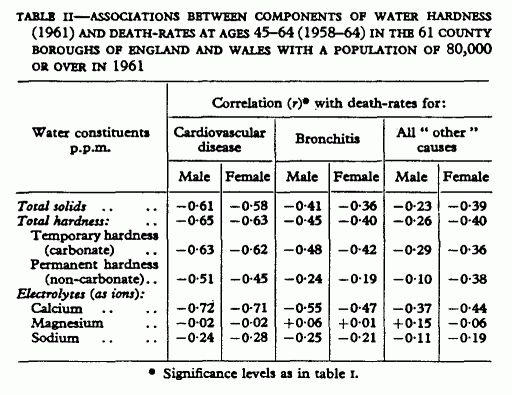
similar. Table II shows the correlations of death-rates with the
various chemical components of water hardness. As in the 1951
series, the highest associations of the cardiovascular rates are
with temporary hardness (i.e., the carbonate fraction) and
calcium; those with sodium much smaller, and with magnesium
negligible. Water hardness and calcium are, of course, highly
correlated (r=0·95) and the correlations of
mortality with water calcium are similar to, though usually
higher than, those with total waxer hardness. The calcium content
of drinking-water would seem, therefore, to be the relevant
component; and since it is a more meaningful entity, and its
estimation is more precise than that of total hardness, calcium
is used in the further analyses.
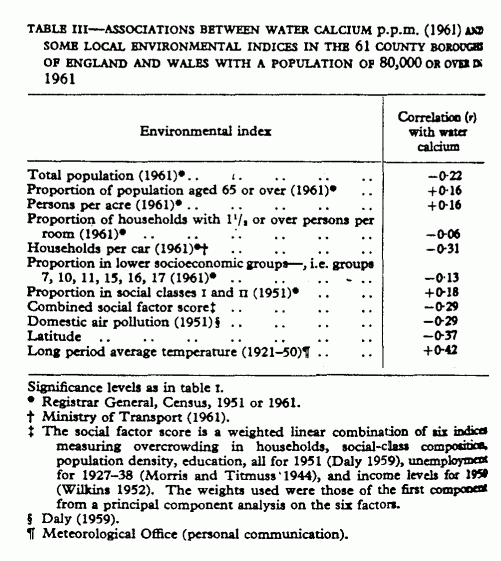
As previously (Morris, Crawford, and Heady 1961), we have
looked for any confounding factor in these associations. Over
sixty social and environmental indices, drawn from Census data,
were correlated with calcium to investigate the possibility that
the correlations between mortality and water calcium (or
hardness) are indirect, and reflect only their mutual association
with other factors. Table III shows a sample, including and
reflecting overcrowding, poverty, climate, and so on, all known
to influence mortality; six of the classic factors associated
with high mortality in general were combined into a social
factor score which can be considered to reflect
socioeconomic conditions in these towns. All of the correlations
with social indices are small, though some are statistically
significant. There are relatively high correlations between water
calcium and both latitude and period temperature; these two
indices are, of course related (r=-0·78) That
death-rates are higher in the North is well known, and both
climatic and social factors contribute. For cardiovascular
disease, however, latitude (or temperature) “effect" seems
to be independent of the water “effect” (Morris,
Crawford and Heady 1962). Furthermore none of the environmental
factors correlated with cardiovascular mortality to the same
degree as did water calcium, and, it is safe to say, not one of
all the indices included could possibly be producing the high
correlations between drinking water and mortality. As in the 1951
series, we have found no indication that water calcium (or
hardness) is merely reflecting any other environmental condition
that we have been able to identify.
Multiple Regression Analysis
Correlation analysis is useful in exploring a problem such as
this. The correlation coefficient measures how closely variation
in one factor is related linearly to variation in another, and
neither factor is assumed to be “dependent“ on
changes of the other. However, having established a relationship
between factors, and postulated which one may be affected by
variation of the other, it is of interest to investigate how
large this effect may be; the method of linear regression is
applicable. Extending this argument and using multiple regression
analysis, the relative contributions of several factors to the
variation of, for example, a death-rate can be estimated. Table
IV shows standardised regression coefficients of death-rates as
three variables: water calcium, social factor score (described in
table III), and latitude. Broadly, these three variables may be
considered to represent water hardness, socioenvironmental, and
climatic factors. Together (last column of table IV) they explain
a high percentage of the variance between the towns of
death-rates for both males and females. The coefficients measure
the “effect” on the death-rate of each factor
separately when the associations with the other two have been
taken into consideration. Water calcium makes a high significant
contribution to cardiovascular mortality and a smaller but still
significant contribution to bronchitis mortality. Its
contribution to “other” male death-rates is not
significant, but to “other” female death-rates it
makes a significant contribution. The contributions of social
factors to the explanation of the variance of cardiovascular
mortality is small, but much higher for bronchitis and the rest
of the non-cardiovascular mortality. Latitude again seems to have
some slight effect (statistically significant at the 5% level) on
cardiovascular and bronchitis mortality when water calcium and
social factors have been allowed for; this is due, perhaps, to
the climatic component. This regression study is focused on
cardiovascular mortality, and a broader study of mortality and
environment in general will be published elsewhere.
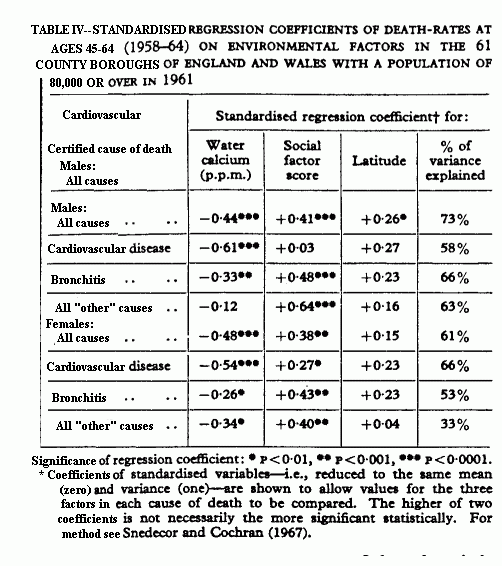
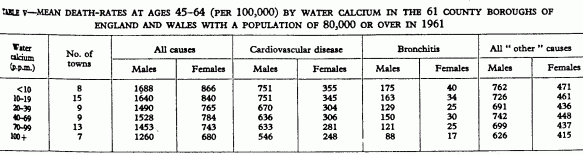
Table V illustrates the decline in mortality associated with
increasing calcium content of drinking-water in the sixty-one
towns. The fall in cardiovascular death-rates is numerically
greater and more consistent than that in bronchitis and
“other” death-rates, although it is proportionally
greater for bronchitis.
Water Chemistry
Since there is no indication that water is merely reflecting
some other factor (though a search for this goes on), the
possibility of a direct relationship must be considered and
thought given to possible mechanisms. Several suggestions can be
made: soft waters could be carrying toxic elements from pipes or
soil into supply; hard waters could be protective due to their
mineral content; trace elements could be involved. A little
progress has been made in investigating these possibilities.
Two separate chemical studies of elements in drinking-water
were made by the Atomic Energy Authority and the Government
Chemist in representative samples of water from consumers’
taps in towns with very soft water and in towns with very hard
water. The results were similar. Analyses were made by emission
spectroscopy and flame photometry of twenty-one elements,
including all likely metal contaminants from water pipes, and
trace elements such as cadmium, vanadium, selenium, and
molybdenum about which there is evidence of some biological
importance even at low concentration.
Possible metal contaminants, copper, zinc, lead, manganese,
and iron were all present in concentrations well below the
maximum allowable limits set by the World Health
Organisation’s (1963) International Standards for Drinking
Water (Crawford and Morris 1967). Mean concentrations of iron,
zinc, lead, tin, nickel, molybdenum, cadmium, chromium, and
indium were similar in the two groups of waters. Mean
concentrations of copper, selenium, and vanadium in the hard
waters were greater than in the soft waters, but this was not
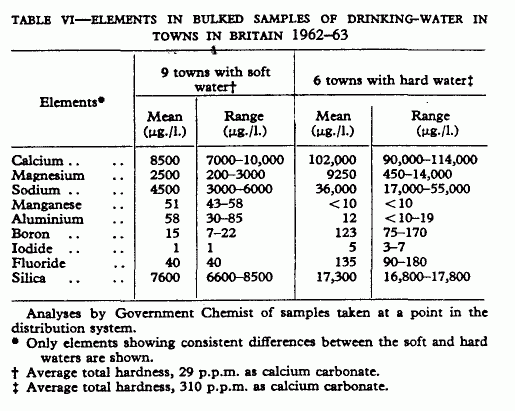
consistent in all samples. Table VI shows mean values of those
elements which did show consistent differences between the very
hard and very soft waters.
Discussion
This second study of mortality in the large towns of England
and Wales confirms the earlier finding of an association between
cardiovascular death-rates and softness of drinking-water, and
again, there is no indication that water is merely reflecting
some third factor. The correlations are higher than in previous
studies and the variation in death-rates over the range of water
hardness is substantial; since cardiovascular disease accounts
for more than a third of all deaths in middle age in the U.K., a
very large number of deaths is involved. A rough estimate of the
number of extra deaths in the sixty-one county boroughs
associated with differences in water calcium, taking the
death-rates of the very hard water areas as baseline, is 2000 per
annum for men aged 45-64— i.e., about one-fifth of the
deaths—assuming water calcium to be distributed similarly
in England and Wales as in these sixty-one county boroughs, this
would involve roughly 8000 deaths per annum in men of this
age.
The striking similarity of the association between water
hardness and mortality in the U.S.A. and U.K. is strong evidence
of a “water factor” in cardiovascular disease. Both
these countries have the necessary conditions for such a factor
to be demonstrated—i.e., large aggregates of population
living at different levels of water hardness, a wide range of
water hardness, and valid death certification at least for the
45-64 age-group (Heasman and Lipworth 1966). All these conditions
have not been present in the studies in Oklahoma and Ireland in
which an association was not found (Lindeman and Assenzo 1964,
Mulcahy 1964); though in Sweden, with small population
aggregates, highly significant associations between
cardiovascular mortality and water calcium were in fact found
(Biorck et al. 1965); in Holland associations were found in women
only (Biersteker 1967). It seems unlikely that the same local
factors associated with cardiovascular mortality could be
producing indirect correlations with water minerals in several
countries. It would seem, therefore, that a “water
factor” in cardiovascular mortality exists, although its
nature and mode of action still escapes us.
It is important to emphasise that the associations found are
with all cardiovascular disease, including cerebrovascular
disease, and not with ischemic heart-disease only, although
deaths from this cause form the biggest fraction. There are many
components of ”cardiovascular disease” —e.g.,
mural atheroma, intravascular thrombosis, hypertension, and so
on. The association could be with any one of these or even with a
non-specific element in cardiac failure. Crawford and Crawford
(1967) in comparing cardiac lesions found more ischemic
myocardial disease in men who had died in a very soft water area
than a very hard water area; but there was no increased
prevalence of coronary atheroma or stenosis in the soft-water
area. Such findings suggested an increased susceptibility of the
myocardium in the area with soft water.
In the material described here the association of water
hardness and cardiovascular disease is not
“specific“— i.e., death-rates from
non-cardiovascular causes are also negatively associated with
water hardness, though to a smaller degree. The consistency of
the higher association found, however, is remarkable considering
the nature of the data and the fact that the components of
cardiovascular disease, mentioned above, are not present
exclusively in deaths certified to cardiovascular disease (Morris
and Crawford 1958). The loss of specificity is greater in the
findings for women.
A possibly important finding, not previously mentioned is a
highly significant association between infant mortality and water
hardness; the correlation (r= -0.55 is much higher than
that found in the 1951 series. In the past, high infant mortality
has been used as a crude index of bad social and health
conditions, but with the general rise in the standard of living
and great expansion of maternity and other health services, the
association between infant mortality and social conditions is
considerably less (Martin 1967). It could be that infant
mortality is reflecting some factor adverse to health which is
correlated with water hardness and which we have not included in
the study; or it could be that water minerals are important in
infant mortality—a "biological” factor showing up as
“social” factors become less important (Morris 1967).
Correlations between water hardness and perinatal mortality,
postneonatal mortality, and stillbirth are -0·35,
-0·44, -0·18, respectively. These associations are
being investigated further.
It is not possible to make detailed comparisons between the
trace elements studies reported here and those of Schroeder
(1966) in the U.S.A., because of differences in sampling. In the
U.K., bulked very soft and very hard waters were studied (mean
total hardness 29 p.p.m. and 310 p.p.m., respectively); in the
U.S.A., Schroeder (1966) compared mean concentrations of
constituents in water from twenty-five cities with the lowest and
twenty-five cities with the highest death-rates from
arteriosclerotic heart-disease in White males 45-64 (mean total
hardness 80 p.p.m. and 139 p.p.m., respectively). The main
similarities and differences of the two studies, however may be
of interest.
In both countries soft waters contained less of each of the
three main elements, calcium, magnesium, sodium, as might be
expected. Elements showing similar differences between soft and
hard waters in both countries are manganese, vanadium, and
silicon—manganese being at a higher concentration in soft
waters and positively correlated with death-rates from
arteriosclerotic heart disease in the U.S.A., and vanadium and
silicon being at higher concentrations in hard waters and
negatively associated with death-rates in the U.S.A. Copper
aluminium are distributed in soft and hard water differently in
the U.S.A. and the U.K.: copper being at a higher concentration
in hard than soft water in the U.K., the reverse being found in
the U.S.A.;.aluminium being at a higher concentration in soft
than hard water in the U.K. but not in the U.S.A. In both
countries the mean concentrations of iron, lead, molybdenum, and
chromium were similar in soft and hard waters.
A high cadmium/zinc ratio in kidneys of rat and mouse has been
found to be associated with arterial hypertension (Schroeder et
al. 1967) but in the waters analysed for us the cadmium
concentration was <0·2 µg. per litre for all soft
and hard water examined; there was little difference in zinc
concentration between soft and hard waters.
In neither Schroeder’s (1966) series nor ours was there
any element present in concentrations which could be toxic in
conventional terms. However, plumbosolvency may still be a
problem in England and waters lying in contact with lead piping
for some (Crawford and Morris 1967). Further study of the
implications, past and present, of plumbosolvency in the U.K. is
now being made. It seems unlikely, however, that lead
contamination could be the whole explanation of the “water
story” in cardiovascular disease, but it could be an
enhancing factor and might explain the much clearer picture and
the higher correlations found in the U.K.
The pH of the water could be important, but we have not
examined this point because of variations, daily and seasonal,
and because of adjustments, by various means, carried out by some
water authorities.
The possibility remains that the water minerals themselves may
be involved. In England and Wales and Sweden the highest
correlations have been with calcium. There was no association
with magnesium in England and Wales or in Sweden, but this, of
course, does not vitiate the possible importance of magnesium in
cardiovascular disease—it merely means that the amount in
drinking water is ineffective, as one might expect considering
the the quantities in water compared with food. On the other
hand, hard drinking-water may provide a meaningful amount of
calcium to the diet (Murray and Wilson 1945). Widdowson (1944)
showed that about a fifth (200 mg.) of the daily calcium
requirement may be obtained by drinking hard water. Widdowson and
McCance (1943) estimated that in some localities the calcium in
drinking-water could be as important a source of calcium as the
foods generally considered to be the main source of this mineral,
a fact usually ignored in assessing calcium intakes. Crawford and
Crawford (1967) found lower average values for calcium and
magnesium in the coronary arteries of young men in a soft-water
area compared with those in a hard water area; they suggested
that the calcium in drinking water, being in ionised form, could
be more readily absorbed and so might make an important special
contribution.
Experimental work is increasingly providing evidence for the
involvement of calcium and magnesium in electrochemical exchange
at cell surfaces associated with changes in adhesion of
cells—e.g., endothelium, platelets, and so on (Hughes and
Tonks 1965, Curran 1967, Durlach 1967)—and in the
myocardium (Bajusz 1965, Nayler 1967), which could be relevant to
the present problem.
Our findings do not clarify the problem of the statistical
association of mortality and the minerals in drinking water; they
do, however, support the suggestion that there is a "water
factor” which makes a material contribution, particularly
to cardiovascular mortality. It may be that we should be thinking
of water as a general factor in mortality operating through the
cardiovascular system and therefore more noticeable in
cardiovascular (and bronchitis) death-rates. The mechanism may be
in the development of heart-failure; incidence and prevalence
studies as well as mortality are required to indicate whether the
associations of water are with disease or a mode of death. There
is urgent need of further studies, individual and ecological
(Morris 1967), and the formulation and testing of useful
hypotheses for which we must wait on the chemists.
We thank the water authorities of the county boroughs and
their chief officers, the United Kingdom Atomic Energy Authority,
the Government Chemist, the General Register Office and the
Meteorological Office. Mrs. R. Glass and Mr. W. Scott of the
Centre of Urban Studies kindly provided us with useful abstracts
of Census data. We also thank our colleagues in the social
medicine research unit, in particular Dr. J. A. Heady for advice,
Mrs. D. Greystoke for clerical help, and Miss P. Ward for
computational assistance.
Requests for reprints should be addressed to M. D. C., Social
Medicine Research Unit, The London School of Hygiene and Tropical
Medicine, 1 Keppel Street, London W.C.l.
REFERENCES
Bajusz, E. (1965) Nutritional Aspects of Cardiovascular
Diseases. London.
Biersteker, K. (1967) Tijdschr. soc. Geneesk.
45, 658.
Biorck, G., Bostrom, H., Widstrom, A. (1965) Acta med.
scand. 178, 239.
Crawford, M. D., Morris, J. N. (1967) Lancet, ii,
1087.
Crawford, T., Crawford, M. D. (1967) ibid. 1,
229.
Curran, R. (1967) Modern Trends in Pathology. London.
Daly, C. (1959) Br. J. prev. soc. Med.
13, 14.
Durlach, J. (1967) Lancet, i, 1382.
Heasman, M. A., Lipworth, L. (1966) Genera] Register Office,
Studies in Medical and Population Subjects no. 20. H.M.
Stationery Office.
Hughes, A., Tonks, R. S. (1965) Lancet, i, 1044.
Kobyashi, J. (1957) Ber. 0hara Inst. landur Forsch.
11, 12.
Lindeman, R. D., Assenzo, J. R. (1964) Am.J. pubi.
Hlth, 54, 1071.
Martin, A. B. (1967) Urban Stud. 4,
1.
Ministry of Transport (1961) Road Motor Vehicles. H.M.
Stationery Office.
Morris, J. N. (1967) Uses of Epidemiology. Edinburgh.
— Crawford, M. D. (1958) Br. med. J. ii,
1485.
— Heady, J. A. (1961) Lancet, i, 860.
— — (1962) ibid. ii, 506.
— Titmuss, R. M. (1944) Med. Offr,
72, 69, 77, 85; and unpublished data.
Mulcahy, R. (1964) J. Irish med. Ass. 55, 17.
Murray, M. M., Wilson, I). C. (1945) Lancet, ii,
23.
Nayler, W. G. (1967) Am. Heart J.
73, 379.
Registrar General Statistical Review of England and Wales,
1958-64; part I (tables, medical). H.M. Stationery Office; and
unpublished data.
— Census 1951 and 1961, England and Wales; county reports;
housing tables; occupation tables. H.M. Stationery Office.
Schroeder, H. A. (l960a) J. Am. med. Ass.
172, 1902.
— (1960b) J chron. Dis.
12, 586.
— (1966) J. Am. med. Ass. 195,
81.
— Nason, A. P., Tipton, J. H. (1967) ibid.
20, 179.
Snedecor, G. W., Cochran, W. G. (1967) Statistical
Methods.
Widdowson, B. M., (1944) Br. med. Bull.
10, 219.
— McCance, R. A. (1943) Lancet, i, 230.
Wilkins, L. T. (1952) Appl. Statist.
1, 27.
World Health Organisation (1963) International Standards for
Drinking Water. Geneva.
This page was first uploaded to The Magnesium Web Site on
October 9, 2002
http://www.mgwater.com/






