THE MAGNESIUM REPORT
CLINICAL, RESEARCH, AND LABORATORY NEWS FOR CARDIOLOGISTS
Second Quarter 2000
Medications, Alcohol Consumption, and Magnesium: Ensuring
Adequate Intake of Oral Magnesium
TIMOTHY J. MAHER, PHD
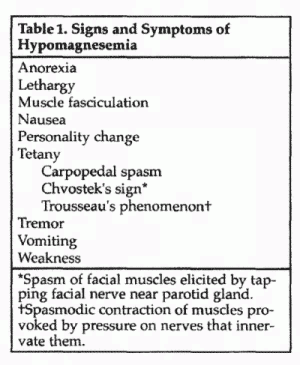
The importance of adequate magnesium intake is documented by
scientific and clinical data that justify the consumption of
magnesium-rich foods and the use of oral magnesium supplements
for good health. When intake or retention of magnesium is
inadequate, magnesium deficiency can take either of 2
forms—clinical hypomagnesemia or chronic latent magnesium
deficiency. Clinical hypomagnesemia is easier to recognize
because it is associated with specific clinical manifestations
(Table 1), but chronic latent magnesium deficiency is more
common.
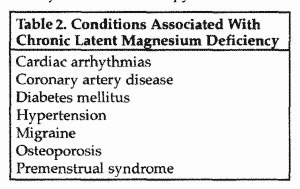
The consequences of chronic latent magnesium deficiency are
extremely prevalent, and some are potentially very serious
(please see The Magnesium Report, First Quarter 2000, in which
Drs. Ronald J. Elin and Robert K. Rude discuss chronic latent
magnesium deficiency and the role of magnesium in preventive
medicine). Chronic latent magnesium deficiency increases the risk
for cardiac arrhythmias, coronary artery disease, diabetes
mellitus, hypertension, migraine, osteoporosis, and premenstrual
syndrome (Table 2).
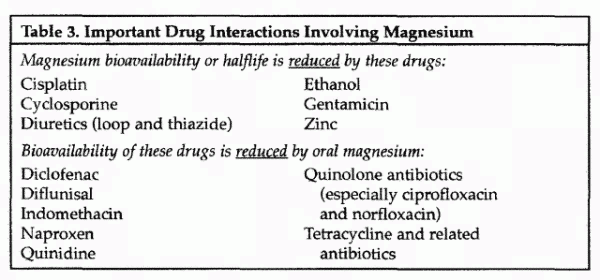
There are clinically significant interactions between
magnesium and a number of medications that may alter magnesium
status and affect medication efficacy (Table 3). Some commonly
used medications, including certain antibiotics, loop and
thiazide diuretics, cisplatin, and cyclosporine can lead to
decreased absorption or increased elimination of magnesium, as
can ethanol consumption. In addition, antacids that contain
magnesium can decrease the bioavailability of some medications by
interfering with intestinal absorption.
Mg absorption and excretion
Magnesium, the second most common intracellular cation in the
body, is a crucial cofactor for more than 300 enzymatic reactions
involved in normal physiologic processes. These include pathways
in metabolism of carbohydrates, fats, and proteins. Magnesium is
required in every enzymatic reaction of ATP and in many of the
steps involved in the synthesis of DNA and RNA, and it stabilizes
these macromolecules as well.
The gut and kidney control the magnesium content of the body.
Magnesium absorption occurs primarily in the small intestine, and
may be impaired by gastrointestinal infections, inflammatory
bowel disease, radiation-induced gastroenteritis, steatorrheic
states, severe diarrhea, and familial malabsorption syndromes. Of
more significance in the gut, however, may be the effect of
magnesium on the absorption or availability of medications.
Normal kidney function is essential to the body’s
ability to retain magnesium. As part of the normal
filtration-reabsorption process, most calcium, magnesium, and
sodium in plasma are filtered through the glomerular membrane.
Unlike calcium and sodium, which are largely reabsorbed in the
proximal convoluted tubule, most magnesium reabsorption takes
place in the loop of Henle—specifically, in the thick
ascending limb of the cortical segment. Some reabsorption also
occurs in the distal. tubule.
Diuretics
Use of diuretics is the most frequent reason for
hypomagnesemia. Magnesium reabsorption in the thick ascending
limb of the loop of Henle is decreased significantly by loop
diuretics, and in the distal tubule by thiazide diuretics. In
addition, patients taking diuretics are at increased risk when
gut absorption of magnesium is compromised by inflammatory bowel
disease, a bout of severe diarrhea, or during an episode of
“stomach flu.” The magnesium-wasting effect of
diuretics is especially important in patients who have cardiac
arrhythmias or risk factors for arrhythmias.
Hypokalemia, which also occurs with use of loop or thiazide
diuretics, may lead to renal magnesium wasting. Cellular
potassium depletion is associated with diminished magnesium
reabsorption within the loop of Henle and the distal tubule and
may lead to increased magnesium excretion. Long-term use of loop
or thiazide diuretics may deplete potassium and promote renal
magnesium wasting.
Most patients who take loop diuretics such as furosemide or
bumetamide or a thiazide diuretic such as chlorothiazide will
become hypomagnesemic unless they augment their magnesium intake
with oral supplements or magnesium-rich foods (Table 4). For
these patients, it is reasonable to emphasize the need for the
recommended daily intake of magnesium—that is, 420 mg/d for
adult men or 320 mg/d for non-pregnant women. A single 400 mg
tablet of magnesium oxide contains 241.3 mg of elemental
magnesium, so 1 or 2 tablets daily is appropriate.

Cisplatin
Cancer patients undergoing chemotherapy with regimens
containing cisplatin are at significant risk for hypomagnesemia.
Cisplatin impairs renal reabsorption of magnesium because it is
directly toxic to the ascending limb of the loop of Henle and the
distal tubule. Approximately 90% of patients who receive this
nephrotoxic antineoplastic drug will become hypomagnesemic unless
corrective pretreatment measures are initiated. Adequate dietary
magnesium intake before chemotherapy will not prevent
cisplatin-associated magnesium deficiency.
While there is general agreement that oral or intravenous (IV)
supplementation of magnesium is appropriate with cisplatin
therapy, there is no consensus on the ideal dosage or mode of
administration. In 4 randomized trials and 2 nonrandomized
studies, a statistically significant benefit for oral or IV
magnesium supplementation has been found. Evans and colleagues
randomized 28 patients receiving cisplatin, 5-fluorouracil, and
epirubicin for upper gastrointestinal cancers to either scheduled
or “as needed” IV magnesium supplementation, with
oral supplementation given if necessary. They found that IV
supplementation alone was not fully protective against
hypomagnesemia during cisplatin chemotherapy but concluded that
patients should receive 1V supplementation with each cycle of
chemotherapy.
Martin and associates found that both oral and IV
supplementation significantly reduced the decline in serum
magnesium levels after the first two or three courses of
chemotherapy. Patients with tumors of the head and neck, ovary,
and bladder were randomized to receive 7.15 mmol of magnesium (as
magnesium pidolate) orally q8h on days 2 through 21 of each
chemotherapy cycle, 12 mmol of magnesium (as magnesium sulfate)
IV in the prehydration fluid given before each cycle, or no
magnesium supplementation (control group). Compared to the
control group, statistically significant differences in serum
magnesium levels were seen after the second cycle in patients
receiving oral supplementation and after the third cycle in
patients receiving IV supplementation.
Ethanol
Persons with alcoholism represent the second largest group of
people with hypomagnesemia. This is due in part to the inherent
effects of alcohol on magnesium homeostasis and in part to the
consequences of the poor diet typical of alcohol abusers.
Acutely, alcohol increases urinary magnesium excretion by as much
as 260% above baseline values; this occurs within minutes of
ingestion or parenteral administration.
With chronic alcohol intake, body stores of magnesium become
depleted. Reasons include inadequate intake, starvation ketosis,
vomiting and diarrhea, and urinary excretion. In advanced
alcoholism, however, urinary magnesium excretion may decline in
response to reduced intake and depleted stores. Among the effects
of chronic alcoholism are a negative magnesium balance, decreased
plasma levels of magnesium, decreased magnesium concentration in
cerebrospinal fluid and in muscle biopsies, and the development
of a magnesium-responsive hypocalcemia.
The increased cancer risk associated with alcoholism may be
partly explained by alcohol-induced magnesium deficiency.
According to Richard Rivlin, MD, of New York’s Memorial
Sloan-Kettering Cancer Center, there are several lines of
evidence suggesting that abnormalities in magnesium metabolism
are associated with cancer development. Among them are the
observations that magnesium inhibits carcinogenesis and may
affect oncogene amplification, and that magnesium deficiency
favors tumor formation by leading to impaired immune surveillance
and enhanced susceptibility of cell membranes to oxidant
injury.
Magnesium repletion by mouth is recommended for patients known
to be heavy drinkers. It is also appropriate to give magnesium
parenterally during alcohol withdrawal. Many of the
manifestations of advanced or chronic alcoholism, such as
personality changes, neuromuscular irritability, seizures, and
delirium tremens, are probably aggravated by magnesium
deficiency. The recalcitrant hypocalcemia frequently seen in
patients with alcoholism usually becomes amenable to treatment
when magnesium is given.
Antacids
A number of antacids contain magnesium, including
AmphojelTM, MaaloxTM, MylantaTM,
and RiopanTM. The liquid formulation of bismuth
subsalicylate, used in the treatment of Helicobacter
pylori infections, also contains magnesium (in the form of
magnesium aluminum silicate, an inactive excipient). These will
not cause hypermagnesemia in the individual with normal renal
function. In the face of renal impairment, however, there can be
significant accumulations of magnesium.
Magnesium, as a divalent cation, can bind with certain drugs,
decreasing their bioavailability (Table 3). Such an effect has
been shown for quinidine, quinolone antibiotics (especially the
older ones such as ciprofloxacin and norfloxacin), tetracycline
and related antibiotics, and certain nonsteroidal
antiinflammatory drugs (specifically diclofenac, diflunisal,
indomethacin, and naproxen). Moreover magnesium loading in the
gut may cause diarrhea, which can adversely affect absorption of
a number of drugs.
Gentamicin
Hypomagnesemia, with concomitant hypocalcemia and hypokalemia,
can develop after less than a week of gentamicin use. A number of
instances of reversible hypomagnesemia secondary to gentamicin
use have been reported. Although the mechanism is unknown,
nephrotoxicity has been suggested, and several investigators have
suggested that gentamicin contributes to renal magnesium and
potassium wasting.
In 1 case report, a 68-year-old woman hospitalized for acute
myelomonocytic leukemia (2 years after being treated with
radiation and chemotherapy for ovarian carcinoma) was given
gentamicin (80 mg q6h) IV when she became febrile. After 7 days
of antibiotic therapy (including penicillin and cefazolin), she
developed Chvostek’s sign and was found to be
hypomagnesemic, hypocalcemic, and hypokalemic. IV administration
of 10 g magnesium sulfate and 120 mmol potassium over 48 hours
was associated with normalization of electrolyte levels and the
disappearance of Chvostek's sign.
In another instance, a 65-year-old man with diabetes and
peripheral vascular disease was given gentamicin (80 mg q8h) IV,
along with clindamycin and metronidazole, after amputation of a
gangrenous leg. After 6 days of gentamicin therapy he developed
Chvostek’s sign and was hypomagnesemic, hypocalcemic, and
hypokalemic. Gentamicin therapy was continued while the patient
was given IV magnesium and potassium replacement. At the end of
the 13-day course of gentamicin, his electrolyte levels were
normal and Chvostek’s sign had disappeared.
Cyclosporine
Magnesium replacement may be appropriate in the treatment and
prevention of hypertension associated with cyclosporine use. In a
case-controlled study, June and colleagues observed that
hypomagnesemia developed in a group of 32 bone marrow transplant
recipients who developed hypertension after cyclosporine
treatment but hypomagnesemia did not develop in 32
cyclosporine-treated controls matched for age, sex, and disease
who remained normotensive. These investigators concluded that
either hypomagnesemia and hypertension were coincident toxic
effects of cyclosporine or that magnesium derangement contributed
to hypertension. Using an animal model, Rob and associates found
that oral magnesium reduced the toxicity of cyclosporine even
when dietary intake of magnesium was normal.
Zinc
The intestinal absorption of magnesium is decreased in persons
taking oral zinc preparations. Zinc is a divalent cation found in
certain cold medication lozenges arid is also taken by some men
for prostate health. Spencer and colleagues studied magnesium
absorption and magnesium and zinc metabolic balance in 142 men
taking high, low, and intermediate levels of calcium. They found
that high zinc intake caused a highly significant decrease in
magnesium absorption and a negative magnesium balance regardless
of calcium intake.
Suggested Reading
Healy DP, Dansereau RJ, Dunn AB, Clendening CE, Mounts AW,
Deepe GS Jr. Reduced tetracycline bioavailability caused by
magnesium aluminum silicate in liquid formulations of bismuth
subsalicylate. Ann Pharmacother. 1997;31:1460-1464.
June CH, Thompson CB, Kennedy MS, Loughran TP Jr, Deeg HJ.
Correlation of hypomagnesemia with the onset of
cyclosporine-associated hypertension in marrow transplant
patients. Transplantation. 1986;41:47-51.
Lajer H, Daugaard G. Cisplatin and hypomagnesemia. Cancer
Treat Rev. 1999;25:47-58.
Nanji AA, Denegri JF. Hypomagnesemia associated with
gentamicin therapy. Drug Intell Clin Pharm.
1984;18:596-598.
Quamme GA. Renal magnesium handling: new insights in
understanding old problems. Kidney Int.
1997;52:1180-1195.
Rivlin RS. Magnesium deficiency and alcohol intake:
mechanisms, clinical significance and possible relation to cancer
development (a review). J Am Coll Nutr.
1994;13:416-423.
Rob PM, Lebeau A, Nobiling R, et al. Magnesium metabolism:
basic aspects and implications of ciclosporine [sic] toxicity in
rats. Nephron. 1996;72:59-66.
Sadowski DC. Drug interactions with antacids. Mechanisms and
clinical significance. Drug Saf. 1994;11:395-407.
Schaafsma G. Bioavailability of calcium and magnesium. Eur
J Clin Nutr. 1997;51(Suppl 1):S13-S16.
Spencer H, Norris C, Williams D. Inhibitory effects of zinc on
magnesium balance and magnesium absorption in man. J Am Coll
Nutr. 1994;13:479-484.
|
The above article is from the "The Magnesium Report",
Second Quarter 2000. Blaine Pharmaceuticals is the
manufacturer of Mag-Ox 400 and Uro-Mag magnesium
supplements. 
Go to Blaine
Pharmaceuticals
|
This page was first uploaded to The Magnesium Web Site on
September 18, 2002
http://www.mgwater.com/





